This is the Weight and Healthcare newsletter! If you like what you are reading, please consider subscribing and/or sharing!
Novo Nordisk (the same company that made a literal fortune by price gouging with insulin,) has taken another step to follow through with their promise to shareholders to generate massive profit from their new weight loss drug Wegovy by getting FDA approval to market the drug to adolescents ages 12 and up.
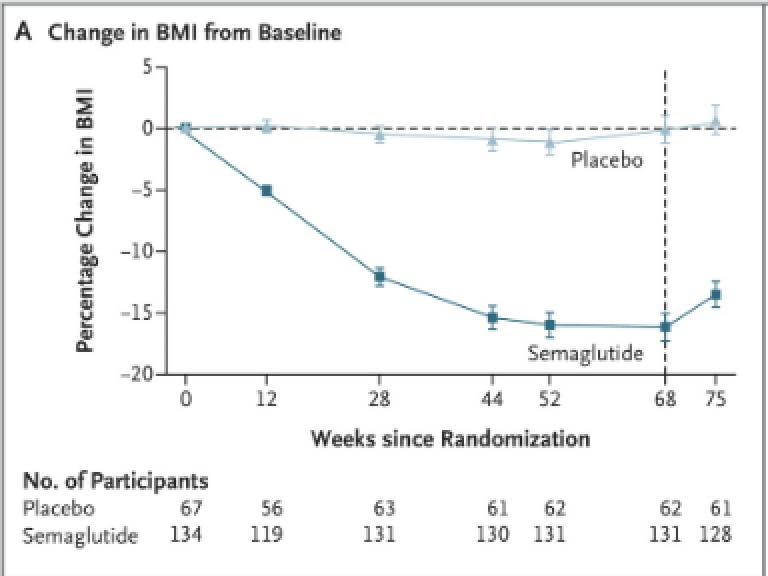
This approval is based on a 68-week trial. They started with 210 participants who were randomized 2 to 1 to get the drug or a placebo, with 180 completing the full 68 weeks.
At week 68, a total of 95 of 131 participants (73%) in the semaglutide (Wegovy) group had weight loss of 5% or more. The youth subjects had greater incidences of gallbladder problems including gallstones, low blood pressure, rash, and itching compared to adults treated. The most frequently reported adverse reactions were nausea, vomiting, diarrhea, headache, and abdominal pain.
There are some obvious things here.
First, according to disclosures, every single researcher on the trial takes money from Novo Nordisk. That’s not proof of impropriety, but it’s certainly something to know (You can find a quick guide to evaluating weight science research here.)
Second, it shouldn’t be shocking that a drug that causes nausea, vomiting, diarrhea, and abdominal pain will also lead to, at least short-term, weight loss. In other studies of similar GLP-1 agonists we have seen that higher amounts of weight loss are predicted by longer onset of gastrointestinal symptoms so there is some question as to how much of the weight loss is the action of the drug on hormones and slowed digestive motility, and how much is just about giving people flu-like symptoms.
Third, at 68 weeks this is a relatively short-term study. In a follow-up to the 68 week adult study of the same drug (Weight regain and cardiometabolic effects after withdrawal of semaglutide: The STEP 1 trial extension,) one year after they had stopped the drug, participants had regained two‐thirds of the weight loss they lost, and lost about two-thirds of their cardiometabolic improvements. This is in line with the significant data showing that almost every weight loss attempt ends in full weight regain within 2-5 years, and it means that, in addition to the side effects of the drug, it may also be exposing those who use it to the risks of weight cycling.
Their follow up from weeks 68 (when the adolescents stopped the medication) until week 75 shows a similar pattern.
Novo Nordisk’s (incredibly profitable for them) answer to the fact that people regain weight after going off the drug, is that they should just stay on the drug, forever. This is a problematic recommendation at best for adults, especially considering it’s only based on a 68-week trial during which participants experienced serious side effects including pancreatitis, gallstones, kidney failure, increased heart rate and depression or thoughts of suicide and a risk of tumors that earned Wegovy a boxed warning – the FDAs strongest warning.
When it comes to children, I think it’s likely far worse. Remember that this is a drug for Type-2 diabetes that was repurposed by Novo Nordisk when they found that weight loss was a side effect and that the market for weight loss drugs was significant. It also allowed them to capitalize on their long-game efforts of having simply existing in a higher-weight body considered a “chronic lifelong health condition” (regardless of actual metabolic health) for which “lifelong treatment” is, they claim, appropriate.
So what happens when you put a 12-year-old (who is far from being done growing) on a large dose of a type 2 diabetes medication with the goal of interfering with their hormones and slowing their digestive system and a risk of serious side effects, and then keep them on it indefinitely?
For Novo Nordisk – massive profits. The sticker price for Wegovy for is $1,350 per month and their behavior around insulin has proven that they are very willing to prioritize profit over human life.
For the patients? Nobody knows. The kids who are prescribed this medication are going to be the ones to find out – very possibly the hard way.
Update: The two-year study of Wegovy gives more insight into the dangers of these drugs. In this study, they break the adverse events into “events per 100 patient years” In terms of total adverse events, the total is 532.3 per 100 patient years. For serious adverse events, it’s 6 per 100 patient years. For adverse events that lead to discontinuation it’s 4 per 100 patient years.
This may not seem like a lot until you think about the fact that when these drugs are prescribed to a 12 year old, even if we assume a life expectancy of just 70 years, someone who starts the drug at 12 could individually have 58 patient-years with an average of 308 total adverse events, 3.48 serious adverse events, and 2.32 events that lead to product discontinuation. (And remember that “produce discontinuation” means, at the very least, weight regain and loss of cardiometabolic benefits based on Novo’s own studies. I do a deep dive into the two-year study here.
As a reminder, there is an option to support the health of fat kids, rather than risking their lives and quality of life trying to make them thin.
Did you find this post helpful? You can subscribe for free to get future posts delivered direct to your inbox, or choose a paid subscription to support the newsletter and get special benefits! Click the Subscribe button below for details:
Liked this piece? Share this piece:
More research and resources:
https://haeshealthsheets.com/resources/
*Note on language: I use “fat” as a neutral descriptor as used by the fat activist community, I use “ob*se” and “overw*ight” to acknowledge that these are terms that were created to medicalize and pathologize fat bodies, with roots in racism and specifically anti-Blackness. Please read Sabrina Strings Fearing the Black Body – the Racial Origins of Fat Phobia and Da’Shaun Harrison Belly of the Beast: The Politics of Anti-Fatness as Anti-Blackness for more on this.




Any time I hear about any type of diet research on children I want to cry. I was in a “study” as a kid and it seriously messed a lot of stuff up for me.
I'll take a look at the conflicts disclosed by the FDA Advisory Board, as well as their minutes. Surprising that a drug with a boxed warning would then be approved for children. Interested also to know if FDA mandated a post-approval study. NN will of course be required to report adverse events, which will be important to follow.