This is the Weight and Healthcare newsletter! If you like what you are reading, please consider subscribing and/or sharing!
In Part 1 and Part 2 we looked at some of the sordid history of diet drugs, which has been a history of the FDA approving these drugs based on studies that were either short-term and/or small (and often in spite of the significant side effects that even these studies showed). The drugs then generate profit for the pharmaceutical companies unless/until they are pulled from the market (often for the exact side effects that were identified in the original studies.) What the drugs don’t do is reliably lead to significant long-term weight loss, as most people lose a little weight and then gain it all back.
In the final part of this series (at least for now) we’ll look at Wegovy and Tirzepatide to see if they appear to be a little bit of history repeating.
The first thing to understand is that, as we’ve talked about before, the weight loss industry (and Novo Nordisk in particular) have been pouring resources into their campaign to declare simply existing in a higher-weight body considered a “chronic, life-long health condition.” On the surface, this simply expands their market into every fat person in existence, but under the surface, it’s more sinister.
One of weight-loss drugs many shortcomings is that people regain the weight after going off the medication (research on Wegovy has already showed rapid regain after going off the drug, with participants regaining about two-thirds of the weight they lost and losing a similar amount of the cardiometabolic gains in the first year after going off the drug.) Since they can’t seem to make the drugs more effective long-term, the goal of this new narrative appears to be people staying on these expensive drugs long-term - even for life. This increases the exposure to harmful side effects for fat people and the profit for the drug companies, but whether or not it creates significant long-term weight loss and/or greater health (which are two different things) and how much harm is done via side effects is still, at best, unknown.
Before I get started I wanted to clarify that the goal of this piece is a surface-level look at the most basic aspects of these drugs, it’s not a deep dive into their research, which is something I’ll likely do in a later piece.
Wegovy
Wegovy is a repurposed diabetes medication called semaglutide (it’s been marketed for diabetes as Ozempic.) It has numerous serious side effects including risk of thyroid c-cell tumors, acute pancreatitis, acute gallbladder disease, hypoglycemia, acute kidney injury, hypersensitivity, diabetic retinopathy complications, heart rate increase, suicidal ideation and behaviors. It carries a Boxed Warning which is the FDA’s most serious warning. That’s in addition to its common side effects which include nausea, diarrhea, vomiting, constipation, stomach pain, headache, fatigue, upset stomach, dizziness, feeling bloated, belching, gas, stomach flu, and heartburn.
Novo Nordisk funded and was deeply involved in their own study, and has let their shareholders know that they are hoping that this becomes a blockbuster drug for them. Marquisele Mercedes already wrote a detailed piece about this for her Patreon and I highly recommend that you read the piece and become a Patreon while you are there! I’ve also written before about Novo Nordisk’s ethically questionable behavior so I won’t go over that again.
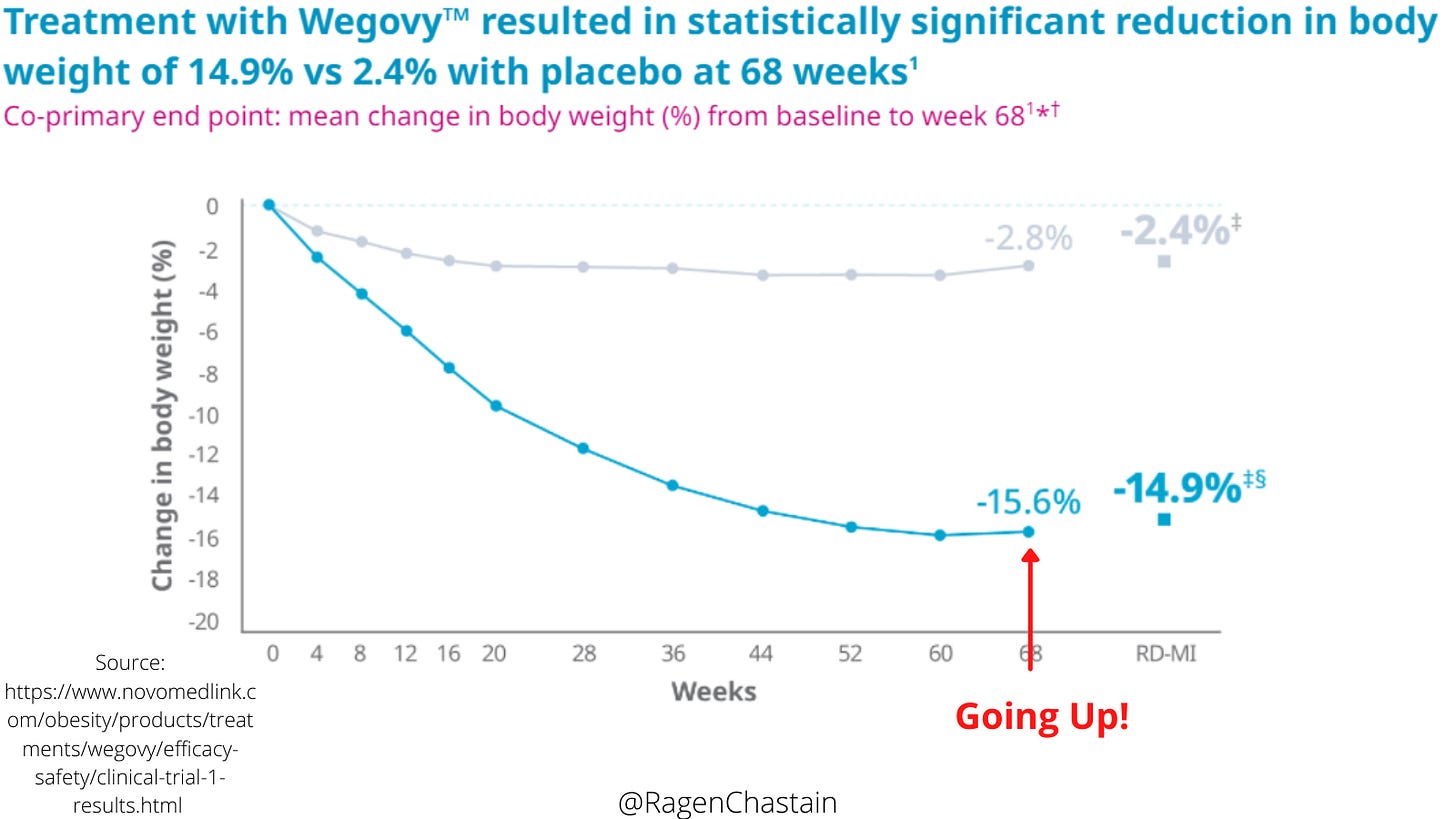
The thing I want to focus on today is their own study results as shown in this graph that they put out (and I annotated in red.)
We know that, by far, the most common outcome of weight loss attempts is short-term weight loss followed by weight gain. (We also know that this weight-cycling is independently linked to harm). We also know that a common tactic in weight-loss research is to utilize a short time frame, capturing the weight loss but not the weight regain.
My interpretation of this graph is that it certainly seems to fit that pattern. There is rapid weight loss at the beginning, then it levels off and ticks up slightly at the end, then the tracking stops. So it’s possible that even their plan to keep people on this drug long-term won’t prevent them from weight regain, all while risking harm from the common and serious side effects of the drug. This is even more concerning considering that Novo Nordisk has already been caught trying to downplay the harm of a similar drug (seriously, read Mikey’s piece!)
Tirzepatide
Tirzepatide is a very similar drug (including similar side effects and an FDA Boxed Warning) manufactured by Eli Lilly. It is currently being marketed under the brand name Mounjaro as a medication for Type 2 Diabetes, they are seeking FDA approval for weight loss. The study they are using to promote it as a weight loss drug was paid for by Eli Lilly, the lead author, Ania Jastreboff, conducts multi-center trials with Eli Lilly, and serves on scientific advisory boards for Eli Lilly, and has accepted thousands of dollars in consulting fees from Eli Lilly. The data preparation and analysis were run by Wei Fan from Eli Lilly, and the medical writing and “editorial assistance” were provided by Farai Chigutsa from…you guessed it…Eli Lilly.
Let’s look at their graph:
We see a similar pattern to Wegovy, most of the weight is lost in the beginning. Then starts to level off just before tracking stops.
I notice in the marketing of these drugs that terms like “sustained” and “long-term” are being used to describe the weight loss, despite the fact that the studies these claims are based on offer a year and a half (or less!) of data, as if everyone involved got collective amnesia about the fact that past experience has shown that typically weight is regained in years 2-5.
Also, these drugs are already showing very serious side effects and yet they are being aggressively pushed on fat people, including by Novo Nordisk which, again, has promised massive sales to their shareholders. Despite a current shortage of the drug they’ve actually increased their “ob*sity sales” goal for 2025 from $1.69 billion USD to $3.72 billion USD. (The drug is priced at about $1,350 per month and doctors who are taking payments from Novo Nordisk have taken to the media - typically without disclosure of their financial interests - to bemoan the lack of insurance coverage.) I predict that between now and then Novo Nordisk will not be in any hurry to put out studies with longer-term weight loss or side effect information.
As always, there is no comparison made to the health benefits (with far less risk) of weight-neutral health interventions.
Finally, note that while Tirzepatide is not yet FDA approved for weight loss, only for Type 2 Diabetes (though it seems that some healthcare practitioners may be prescribing it “off label,”) the FDA approval for Wegovy as a weight-loss drug predicates risk on body size (allowing prescriptions to people with a BMI 27 kg/m2 or greater who have at least one "weight-related comorbidity" or any patients with a BMI of 30.) So approval (based on a risk/benefit analysis) is based on fatness alone for some patients, and for others based on the idea that health conditions that people of all sizes get are "weight-related." This follows another dangerous pattern of weight -stigma in healthcare - viewing people as less-valuable and their lives more riskable at higher weights.
One of the things that I always have to remember as a fat patient is that pharmaceutical companies have a fiduciary duty to their shareholders, not to me as a patient (and there are plenty of examples of pharmaceutical companies making the decision to harm patients based on their assessment that the penalties/lawsuits will be less than the profits.) And many healthcare providers (including and especially those who work in “ob*sity medicine”) firmly believe that it’s worth risking my life and quality of life in any and all efforts to make me even a little bit thinner. That means that, for me, healthcare is always patient beware. When it comes to weight loss drugs, saying that I am wary is a vast understatement.
Did you find this post helpful? You can subscribe for free to get future posts delivered direct to your inbox, or choose a paid subscription to support the newsletter and get special benefits! Click the Subscribe button below for details:
More research and resources:
https://haeshealthsheets.com/resources/
*Note on language: I use “fat” as a neutral descriptor as used by the fat activist community, I use “ob*se” and “overw*ight” to acknowledge that these are terms that were created to medicalize and pathologize fat bodies, with roots in racism and specifically anti-Blackness. Please read Sabrina Strings’ Fearing the Black Body – the Racial Origins of Fat Phobia and Da’Shaun Harrison’s Belly of the Beast: The Politics of Anti-Fatness as Anti-Blackness for more on this.





This is such a great overview of these drugs. I can speak from experience (fat and T2 diabetic) that these meds are ROUGH! I took the oral version (Rybelsus) of Wegovy/Ozempic and within 5 months on it I had to come off due to suicidal ideations. The GI issues were miserable too, but SI was scary. I’m now on Mounjaro and GI side effects are still there. It’s frustrating.
I am convinced that semaglutide is going to go down with *glitazone diabetes drugs (thiazolidinediones such as Actos, Rezulin, & Avandia) as disasters that cause more problems and deaths than they "helped." Forcing a non-diabetic's pancreas to work harder for no other reason than weight loss is not going to be healthy long term. And of course it makes sense that the effects are only temporary, given that making a non-diabetic have more insulin in the blood stream (or a diabetic having more insulin than needed) is going to promote weight gain, added to the risks of all sorts of health problems caused by the mechanisms itself.
Note that some *glitazone drugs are still available in the US, and other places, though in the US they come with black box warnings for potential congestive heart failure and liver problems, the latter being why Rezulin was pulled off the market.