This is the Weight and Healthcare newsletter! If you like what you are reading, please consider subscribing and/or sharing!
As I discuss the issues of GLP-1 agonist and GLP-1/GIP co-agonist weight loss drugs Semaglutide (Ozempic/Wegovy) and Tirzepatide (Mounjaro/Zepbound)I’ve been increasingly hearing from people who are engaged in practices with these drugs that are not remotely evidence based.
Concerningly, many of these came to me directly from healthcare providers who were engaged in the practices, or from patients whose healthcare providers had recommended them and who then contacted me to ask me about them.
Prescribing these drugs as a “jump start” while people learn about “healthy habits”
This is more magical thinking than evidence-based medicine. The research that exists shows that people regain weight when they go off the drug, period. This is shown through Novo Nordisk and Eli Lilly’s own research in what are called withdrawal studies, in which the study group is given the drug for a certain amount of time and then taken off the drug. I think it’s also important to note that Novo and Eli Lilly were happy to put this out because, even though it shows that they are just like every other weight loss drug, they came upon the massively profitable idea of claiming that this doesn’t clearly show that they are just another failed weight loss drug but, instead, that it proves that people should just stay on these drugs for the rest of their lives.
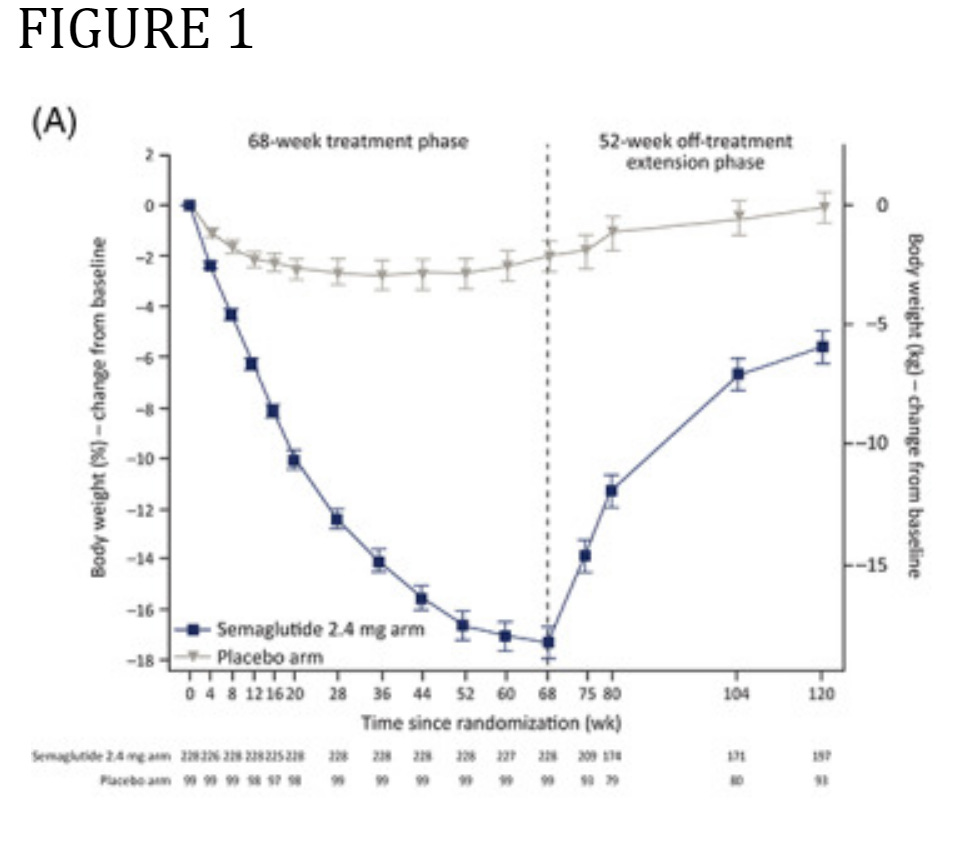
Here is the graph of Wegovy’s withdrawal study in adults (the blue line shows weight loss on the drug for 68 weeks (the dotted vertical line) and weight regain in the 52 weeks after. (Note that weight is still trending up when they stopped tracking.)
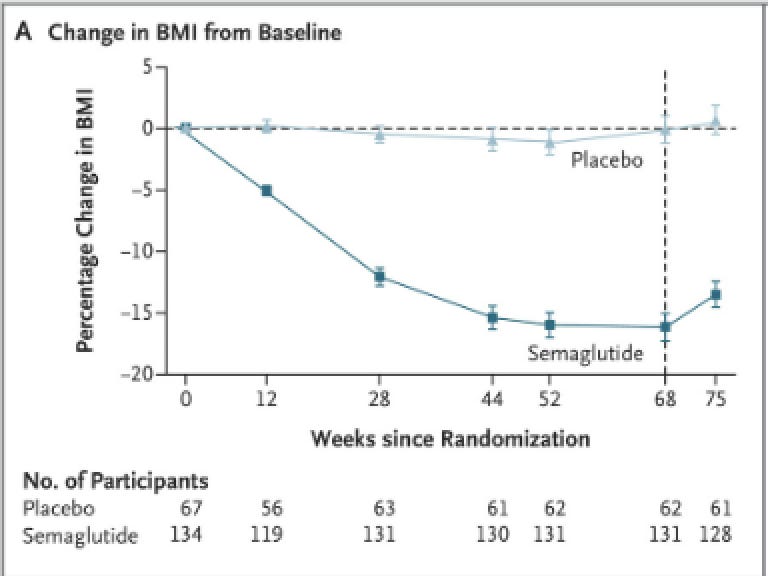
Here it is for the brief time they tracked after kids took Wegovy. Here the weight loss leveled off at about 52 weeks and then regain started at 68 weeks (the dotted vertical line) when the kids went off the drug. I wrote about this study here.
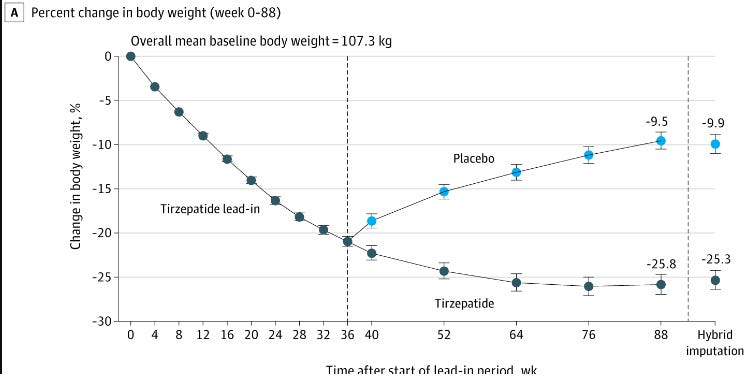
Here is the Tirzepatide (Zepbound) withdrawal study. In this study part of the group stayed on the drug while the other stopped taking it. Again, at 36 weeks the weight loss of those who stayed on the drug slowed down, but for those who went off the drug regain was rapid (and, again, was trending up when they stopped tracking.) I did a deep dive into this study here.
Not only is there no research to suggest that people can maintain weight loss after going off of these drugs, there is no research to suggest that there are any behavior-based weight loss interventions that will lead to long-term significant weight loss (or maintenance of weight loss after going off the drug). The only research we have on behavior-based weight loss interventions is a 100-year track record of abject failure.
The belief that practicing healthy habits will result in significant, long-term weight loss is so persistent that it is treated more like religious doctrine than something that requires a basis in evidence (which it absolutely does not have.) Also, this idea that “healthy habits” will create weight loss maintenance is based on the assumption that fat* people aren’t participating in these habits already. Understanding that health is an amorphous concept and is not an obligation, barometer of worthiness, or entirely within our control, the idea that if someone is fat then they don’t know/practice healthy habits (by whatever definition) is much more weight-stigma than science.
Claiming that once people have lost the desired amount of weight, they can move to a “much lower maintenance dose.”
First of all, remember that weight loss is far from guaranteed, even if people stay on the drug. The prescribing instructions allow for some dosage de-escalations if the drug isn’t well tolerated at the higher doses, but there is no research that shows that people can move to a much lower dose and maintain weight loss.
The initial FDA-approved prescribing guidelines for Wegovy (Novo Nordisk’s Semaglutide) were "Initiate at 0.25 mg once weekly for 4 weeks. In 4 week intervals, increase the dose until a dose of 2.4 mg is reached (2.3). The maintenance dose of WEGOVY is 2.4 mg once weekly. If patients do not tolerate the maintenance 2.4 mg once-weekly dose, the dose can be temporarily decreased to 1.7 mg once-weekly, for a maximum of 4 weeks. After 4 weeks, increase WEGOVY to the maintenance 2.4 mg once-weekly. Discontinue WEGOVY if the patient cannot tolerate the 2.4 mg dose. (emphasis mine)
They have been updated to allow for a 1.7mg dose (though the 2.4mg dose is still “recommended”) and they still say “Discontinue WEGOVY if the patient cannot tolerate the 1.7 mg once-weekly dosage” (emphasis mine)
The prescribing guidelines for Zepbound (Eli Lilly’s Tirzepatide) give a bit more latitude “The recommended starting dosage is 2.5 mg injected subcutaneously once weekly. After 4 weeks, increase to 5 mg injected subcutaneously once weekly. • Increase the dosage in 2.5 mg increments after at least 4 weeks on the current dose. The recommended maintenance dosages are 5 mg, 10 mg, or 15 mg injected subcutaneously once weekly.
It should be made clear, though, that in their more recent studies, anyone who couldn’t maintain at least a 10mg dose was kicked out of the study, those who managed to stay at that dose were regaining weight at 88 weeks, and there is still no research to suggest that people could lower their dosage and maintain weight loss.
Compounding the drugs
Instead of dispensing the commercially available forms of a medication, compounding pharmacies create medications based on the specific needs of an individual patient by mixing, combining or altering ingredients. Critically, while FDA approved is not nearly the same thing as “safe,” compounded drugs are not FDA approved.
Compounded drugs are important to many people for many reasons. Some people may need a dosage of a medication that is lower than what is commercially available, or they may not be able to swallow a pill and thus need a medication compounded into liquid form. Also, many pet medications are created by compounding pharmacies.
The problem with compounding Wegovy is that in some cases they are being made with salt forms of semaglutide that are different than what is actually used to make Novo Nordisk’s drug, including semaglutide sodium and semaglutide acetate. In a letter to the National Association of Boards of Pharmacy the FDA wrote:
“We also wish to ensure you are aware that the active pharmaceutical ingredient in Wegovy and Ozempic is semaglutide in its base form. We are aware that in some cases compounders may be using salt forms of semaglutide, including semaglutide sodium and semaglutide acetate. We are not aware of any basis for compounding a drug using these semaglutide salts that would meet federal law requirements that limit the types of active ingredients that can be used in compounding.”
Micro dosing GLP-1 Agonists
I heard about this one from a patient whose naturopath is offering this service. (Nothing against naturopaths, but lots against any healthcare provider who is providing healthcare without an evidentiary basis.) This may be done using compounded drugs or a lower dose than what would normally be prescribed. I could find no data suggesting that this is either safe or “effective” for weight loss.
Using these drugs to smooth out weight-cycling
Again, the practice of cycling on and off these drugs is not based in any kind of research. They are just learning that these drugs work in the brain, not just the gut as originally expected, in unknown ways and for unknown duration, and adding unstudied intermittent usage creates a number of unknowns. Also, if your weight is increasing, and you take a drug to reduce it, then go off the drug and your weight increases, so you take a drug to reduce it that sounds a lot like weight cycling which is independently linked to significant harm. It’s also unclear how this would work. As I mentioned above, these medications have an escalation schedule that lasts for months - are people who are using these drugs to “smooth out weight cycling” re-initiating and escalating each time? Are they jumping in and out at their max dose? Again there is no research on either practice.
All patients deserve ethical, evidence-based medicine and informed consent, and that includes being informed that a healthcare practitioner or commercial weight loss company (I’m looking at you, Noom) is marketing/recommending/prescribing something that is not remotely evidence-based.
For GLP-1 weight loss meds in particular, Medical Students for Size Inclusivity have create an informed consent project that you can find here!
Did you find this post helpful? You can subscribe for free to get future posts delivered direct to your inbox, or choose a paid subscription to support the newsletter (and the work that goes into it!) and get special benefits! Click the Subscribe button below for details:
Liked the piece? Share the piece!
More research and resources:
https://haeshealthsheets.com/resources/
*Note on language: I use “fat” as a neutral descriptor as used by the fat activist community, I use “ob*se” and “overw*ight” to acknowledge that these are terms that were created to medicalize and pathologize fat bodies, with roots in racism and specifically anti-Blackness. Please read Sabrina Strings’ Fearing the Black Body – the Racial Origins of Fat Phobia and Da’Shaun Harrison’s Belly of the Beast: The Politics of Anti-Fatness as Anti-Blackness for more on this.




I am furious that more research isn't done about gall stones cause by these drugs. I took myself off of Trulicity when I told my PCP that I was experiencing a lot of pain under my right ribcage, and she told me to wait and see if it spread to the back. I called her a few days (and sleepless nights from the pain) later, and said it was now in my back. She said I should go to urgent care and tell them I was on a GLP-1 drug.
So they know. Confirmed by an online conversation with a physician who blithely said "well when we disrupt the digestion we know it will show up as gall stones" or something similar.
They fucking know. And they don't care because fat is worse than debilitating pain and damage to internal organs.
Thank you for continuing to research and interpret the data to provide digestible (oh puns, I can't stay mad at you), understandable information. I'm so disillusioned with healthcare in America. I haven't experienced healthcare elsewhere, it's just so slapped-together and not comprehensive or individualized to be caring enough for me.
I've been tempted to comment for a while now, perhaps with an "ask me anything" approach ("I'm on Ozempic, AMA" or "my doc has prescribed weight loss surgery, AMA" or, or, or...). Those are true statements, but this is your Substack, not mine. Just know this: I appreciate you for so many reasons, and especially for this.
* It amuses me that AMA doubles up here for those in the know: Ask Me Anything: I'm thinking about going Against Medical Advice.
🙌🏼♥️👏🏼